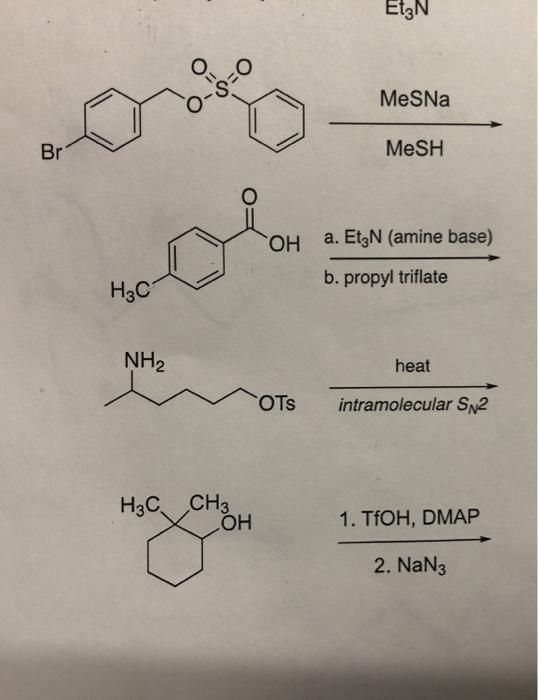
Mechanisms for interaction between acetic acid and triethylamine, (a)... | Download Scientific Diagram
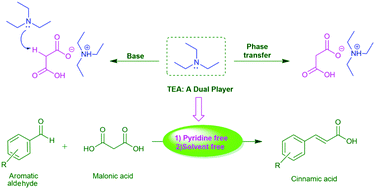
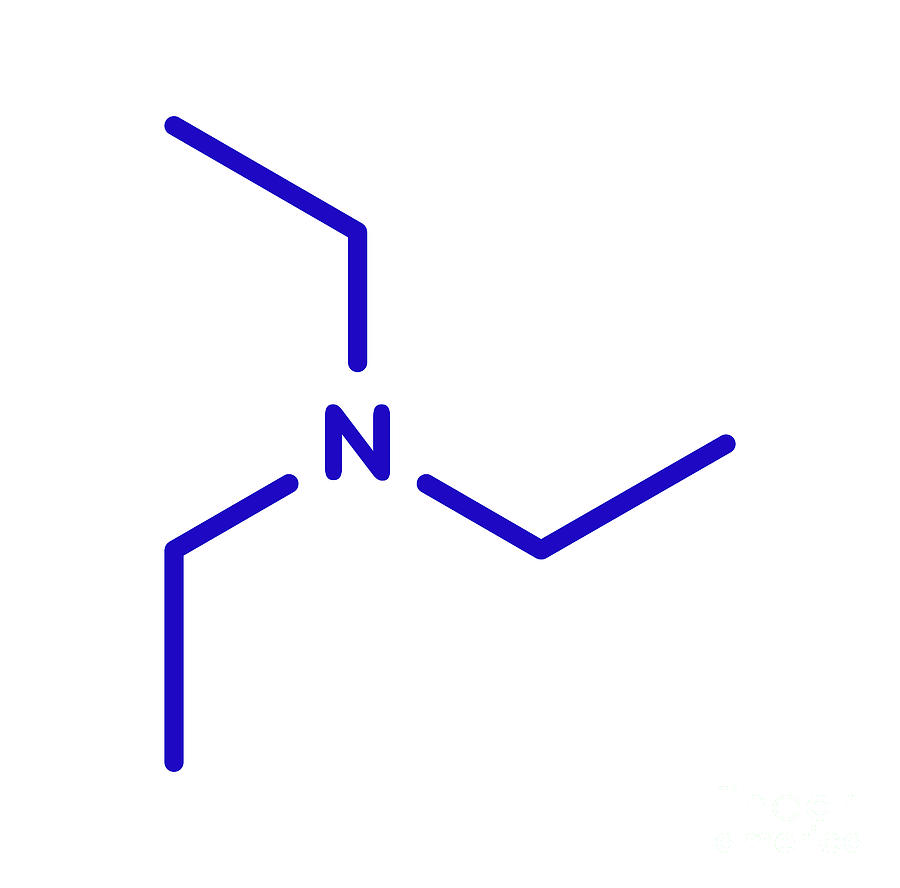
Triethylamine: a potential N-base surrogate for pyridine in Knoevenagel condensation of aromatic aldehydes and malonic acid - New Journal of Chemistry (RSC Publishing)

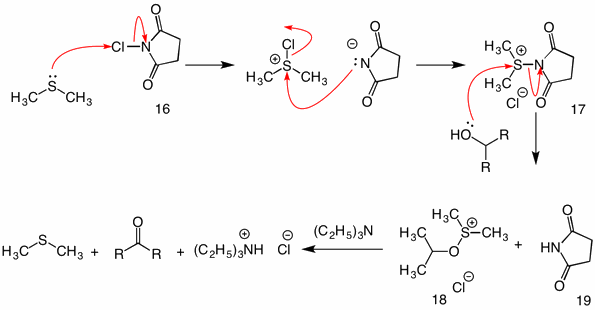
Unprecedented cyclization of α-diazo hydrazones upon N-H functionalization: A Et3N base promoted one-step synthetic approach for the synthesis of N-amino-1,2,3-triazole derivatives from α-diazo hydrazone - ScienceDirect

entry 5). The use of other bases such as Et3N, DIPEA, or NH4OAc did not... | Download Scientific Diagram
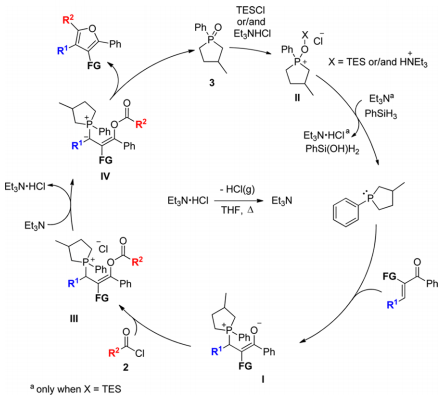
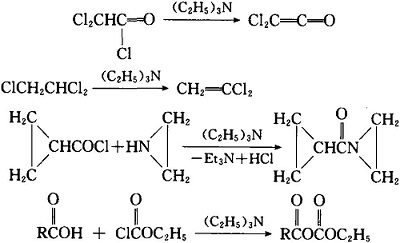
Synthesis of Functionalized Furans via Chemoselective Reduction/Wittig Reaction Using Catalytic Triethylamine and Phosphine

organic chemistry - Why the formation of a fog is observed when triethylamine is added? - Chemistry Stack Exchange

Triethylamine Hydroiodide as a Simple Yet Effective Bifunctional Catalyst for CO2 Fixation Reactions with Epoxides under Mild Conditions | ACS Sustainable Chemistry & Engineering

Triethylamine Hydroiodide as a Simple Yet Effective Bifunctional Catalyst for CO2 Fixation Reactions with Epoxides under Mild Conditions | ACS Sustainable Chemistry & Engineering

Triethylamine Hydroiodide as a Bifunctional Catalyst for the Solvent‐Free Synthesis of 2‐Oxazolidinones - Nishiyori - 2020 - European Journal of Organic Chemistry - Wiley Online Library