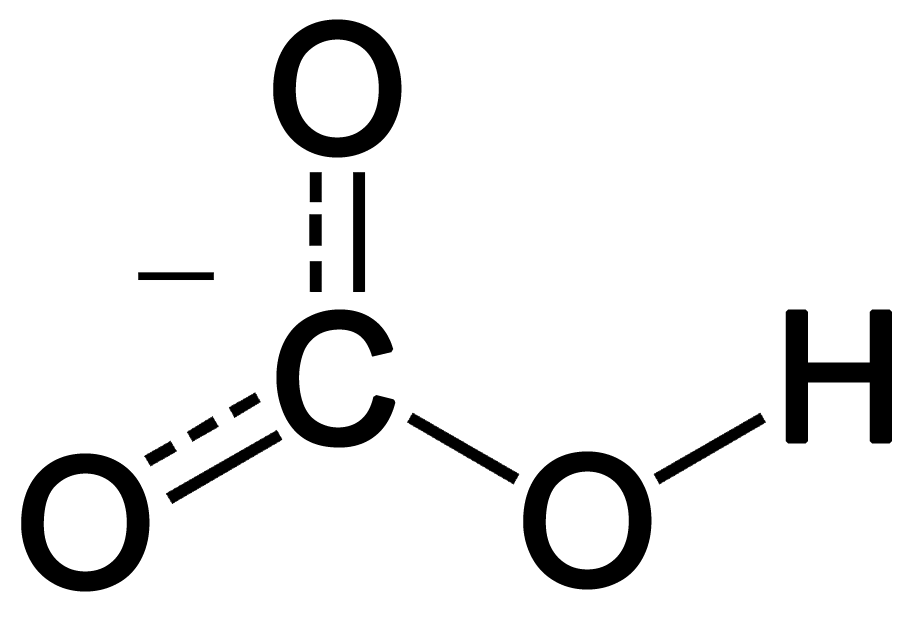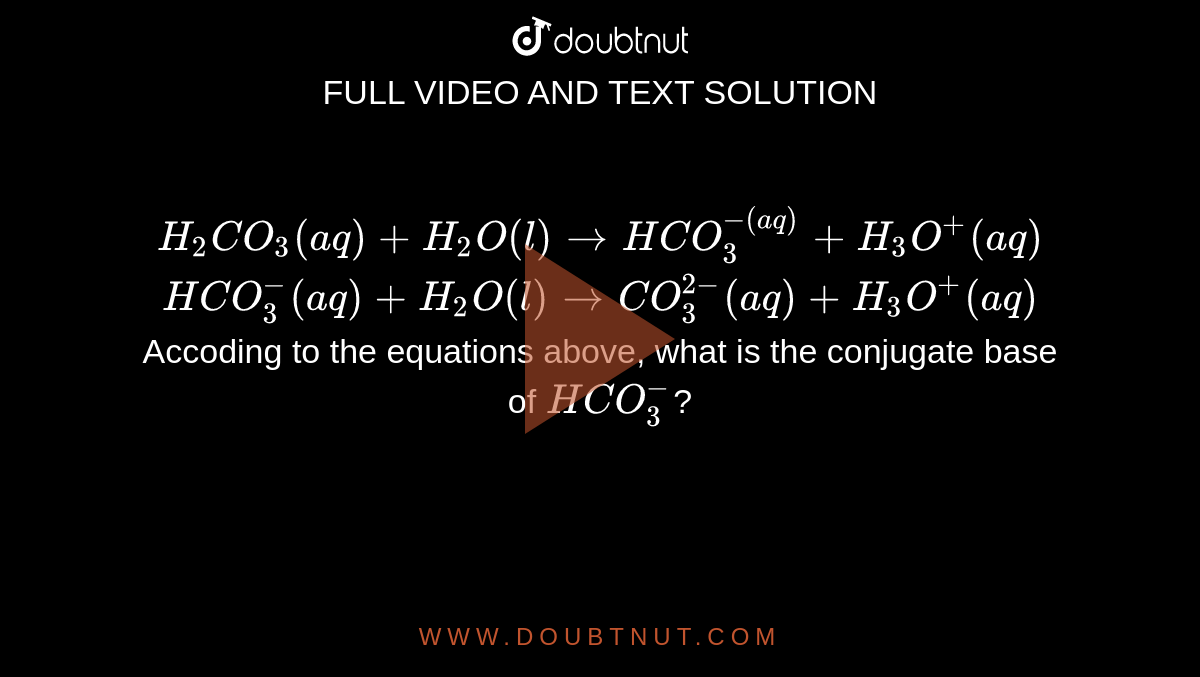
H2CO3(aq)+H2O(l)toHCO3^-(aq) + H3O^+(aq) HCO3^(-)(aq) + H2O(l)to CO3^(2-)(aq)+H3O^(+)(aq) Accoding to the equations above, what is the conjugate base of HCO3^-?
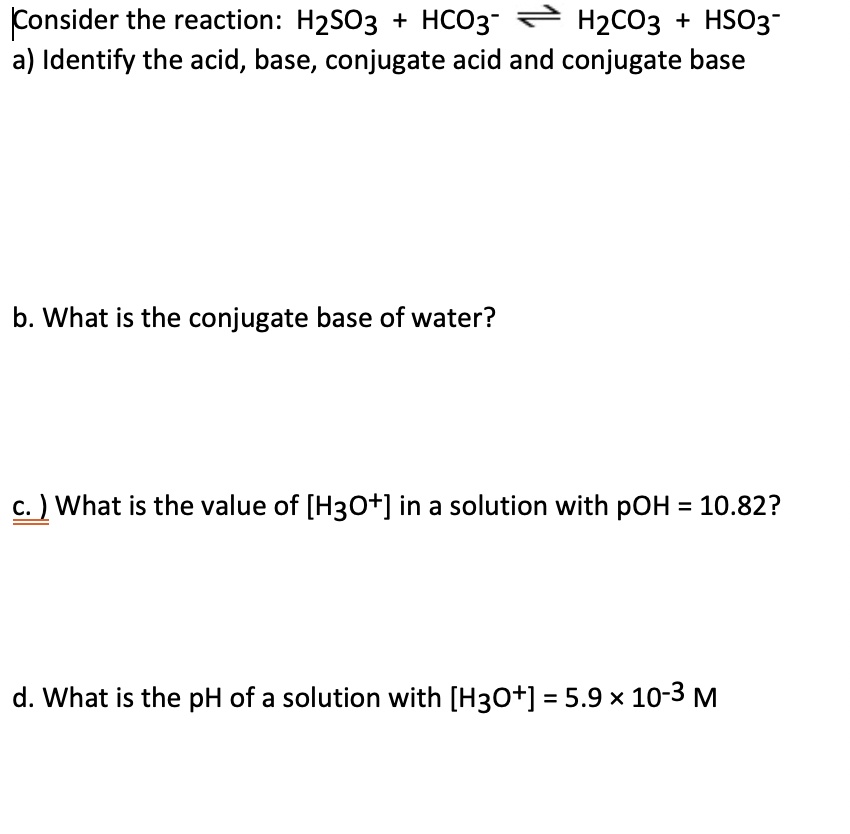
SOLVED: Konsider the reaction: H2SO3 HCO3" H2CO3 HSO3" a) Identify the acid, base, conjugate acid and conjugate base b. What is the conjugate base of water? C What is the value of [

The Importance of Acid–Base Equilibria in Bicarbonate Electrolytes for CO2 Electrochemical Reduction and CO Reoxidation Studied on Au(hkl) Electrodes | Langmuir

Identify the conjugate acid-base pairs in the following reaction. Indicate what each substance is in each pair. H2CO3 + PO43- arrow HCO3- + HPO42- | Homework.Study.com

![pHe/ [HCO3-] (Davenport) diagram of the extracellular acid–base... | Download Scientific Diagram pHe/ [HCO3-] (Davenport) diagram of the extracellular acid–base... | Download Scientific Diagram](https://www.researchgate.net/publication/232279532/figure/fig5/AS:216381174882304@1428600700010/pHe-HCO3-Davenport-diagram-of-the-extracellular-acid-base-variables-Acid-base.png)

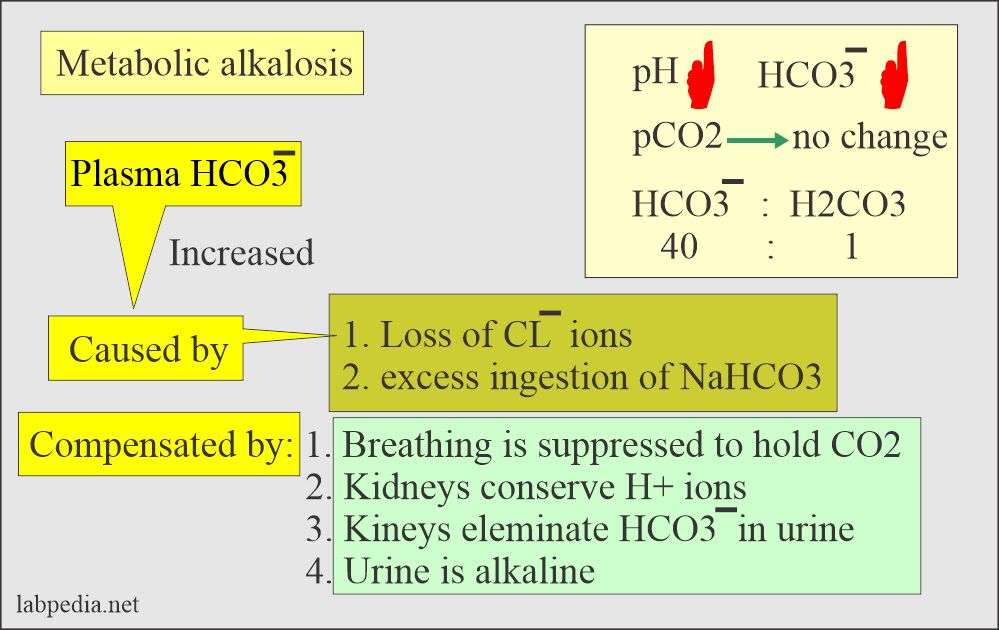



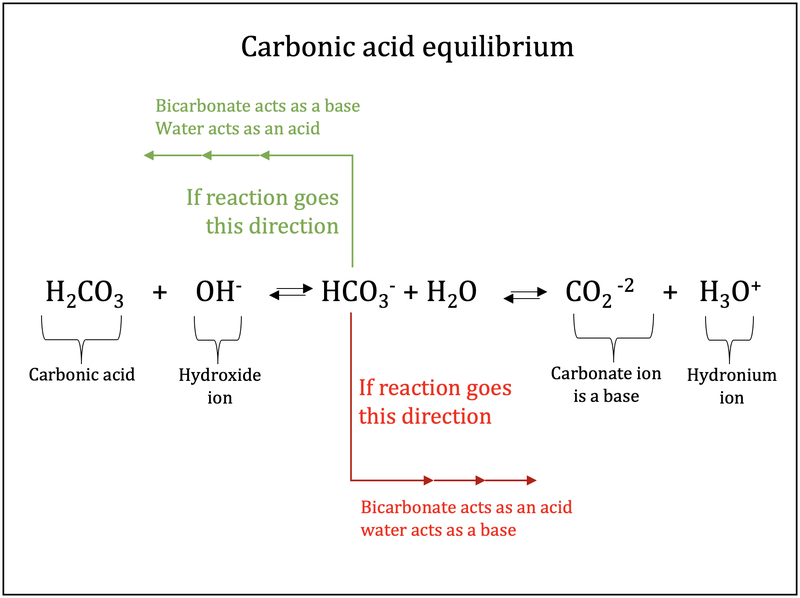

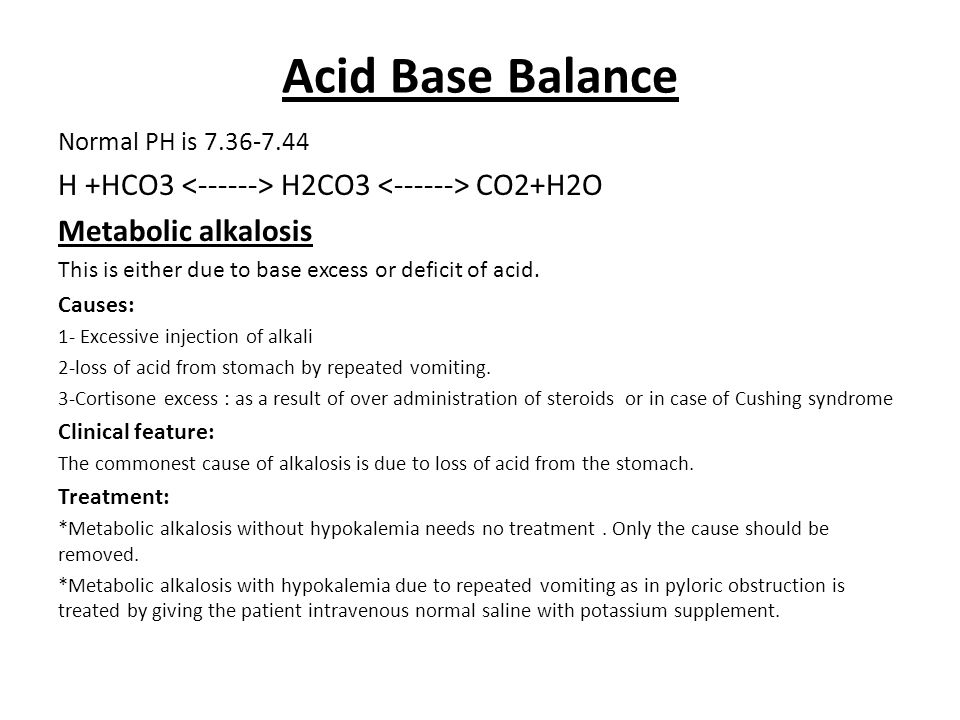

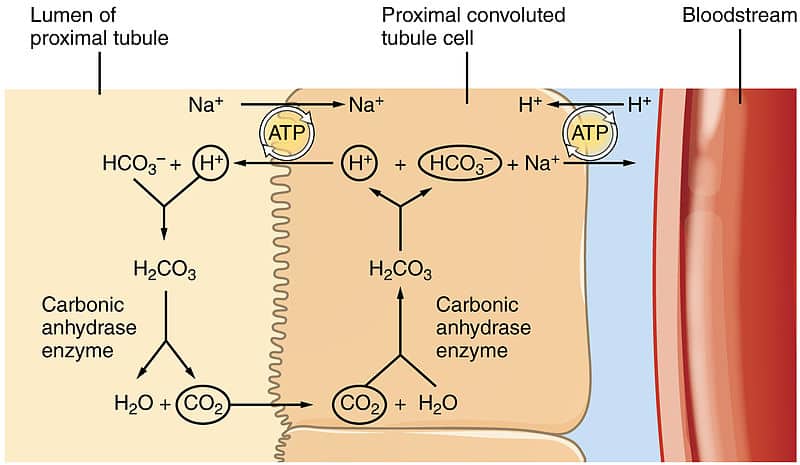
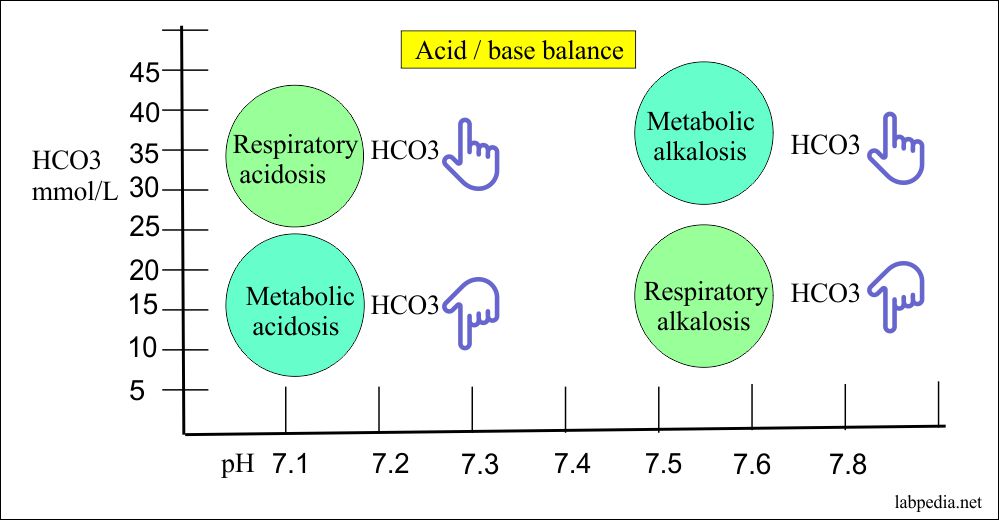
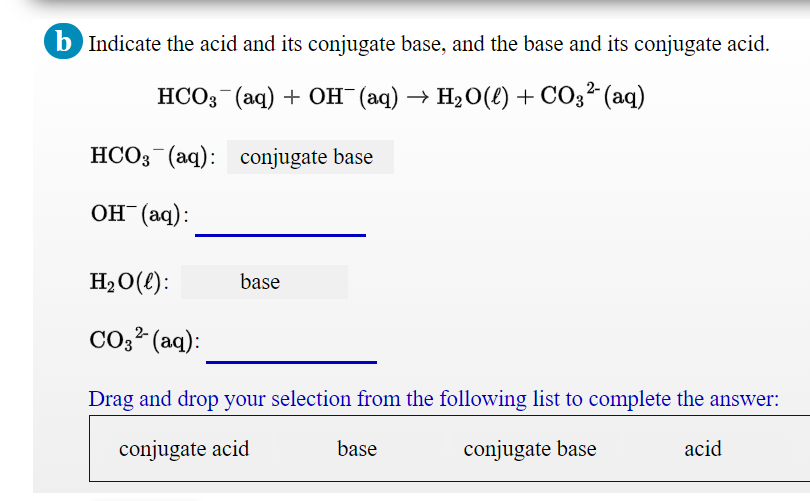

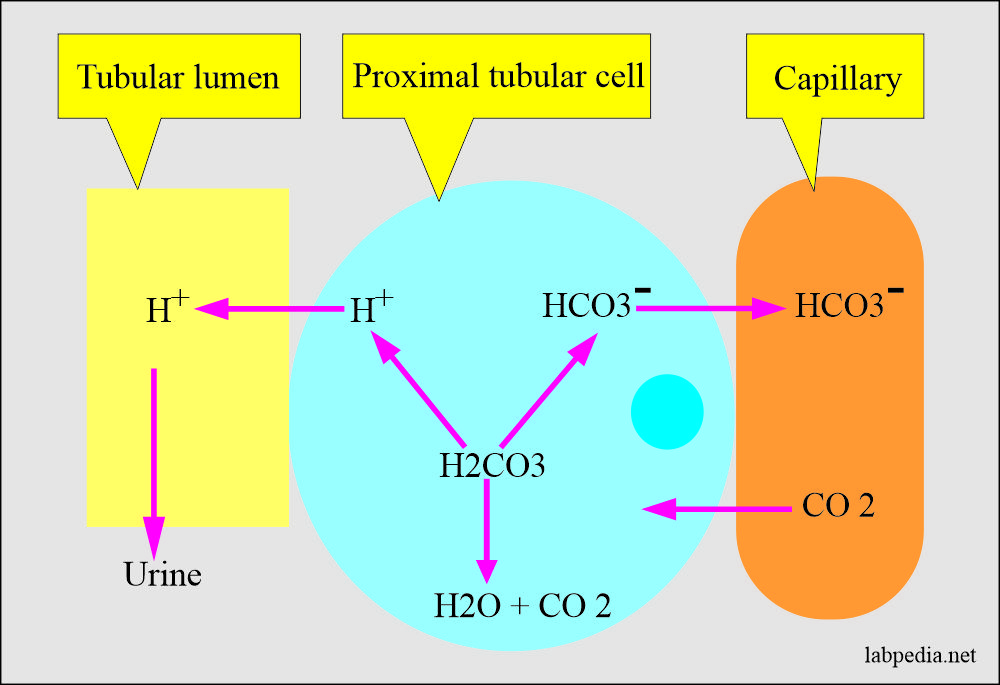


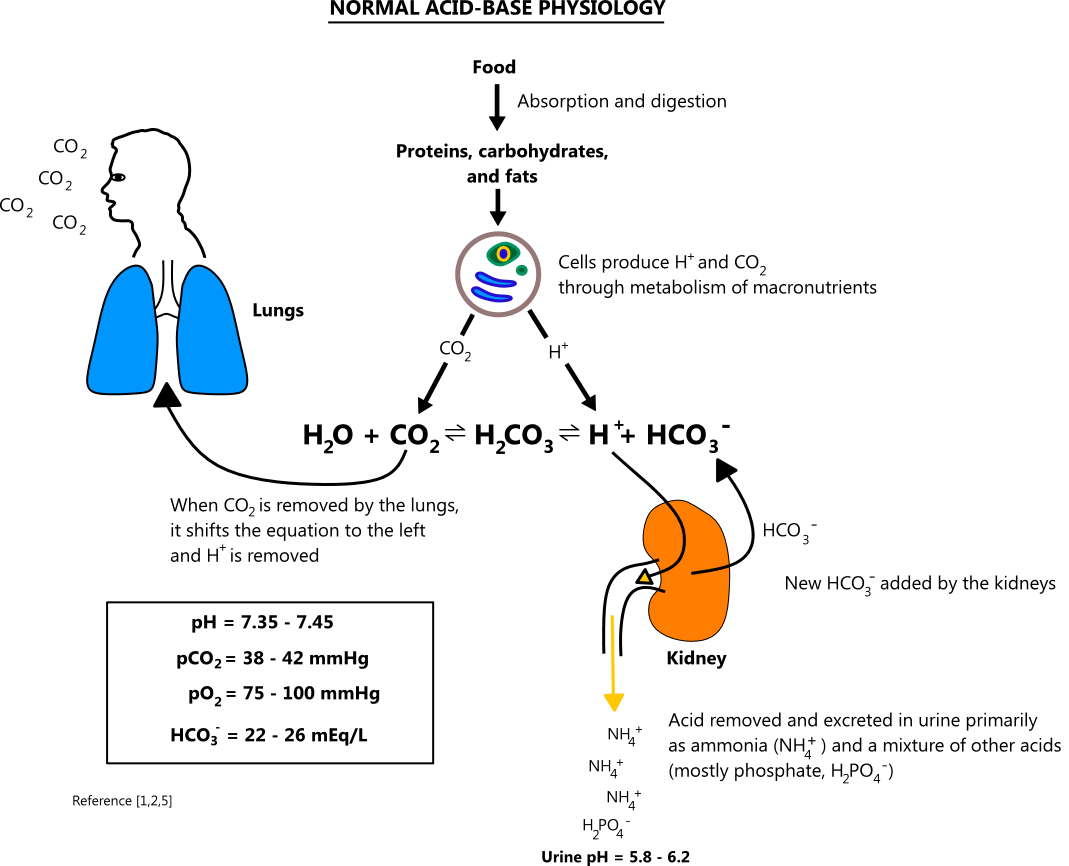
![ANSWERED] In the following reaction: HCO3(aq) + H₂... - Inorganic Chemistry ANSWERED] In the following reaction: HCO3(aq) + H₂... - Inorganic Chemistry](https://media.kunduz.com/media/sug-question/raw/53160915-1659276540.7997923.jpeg)