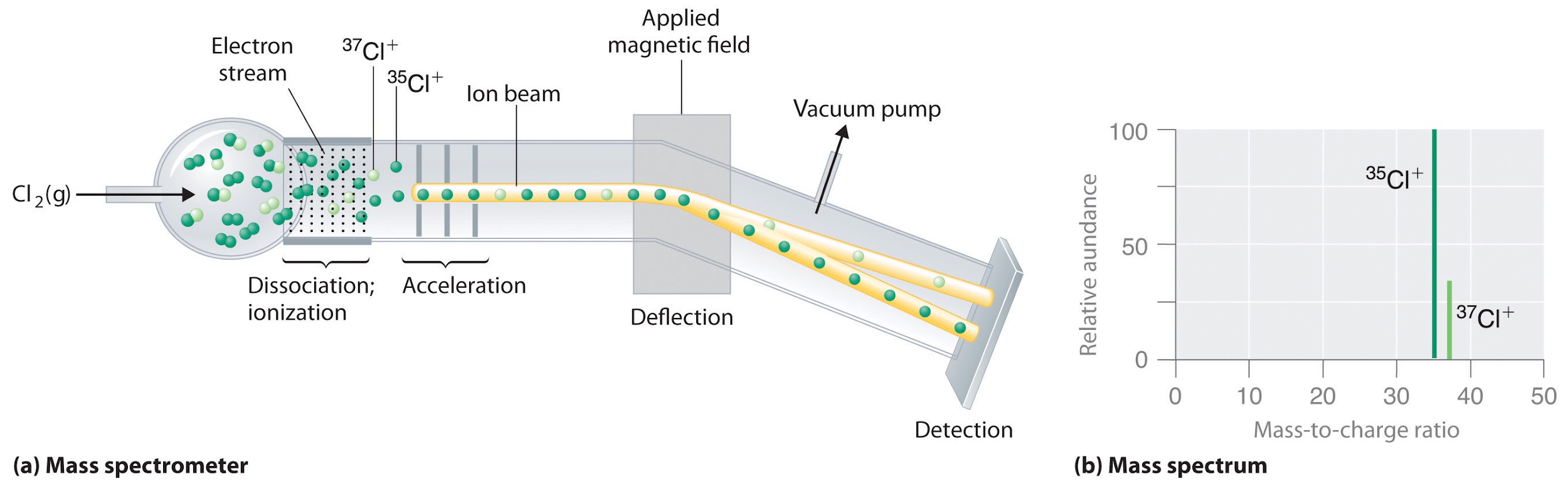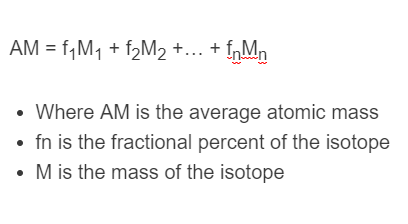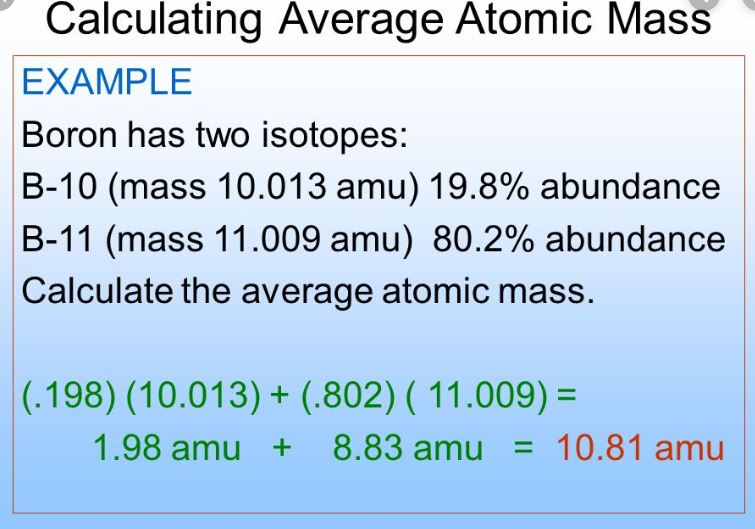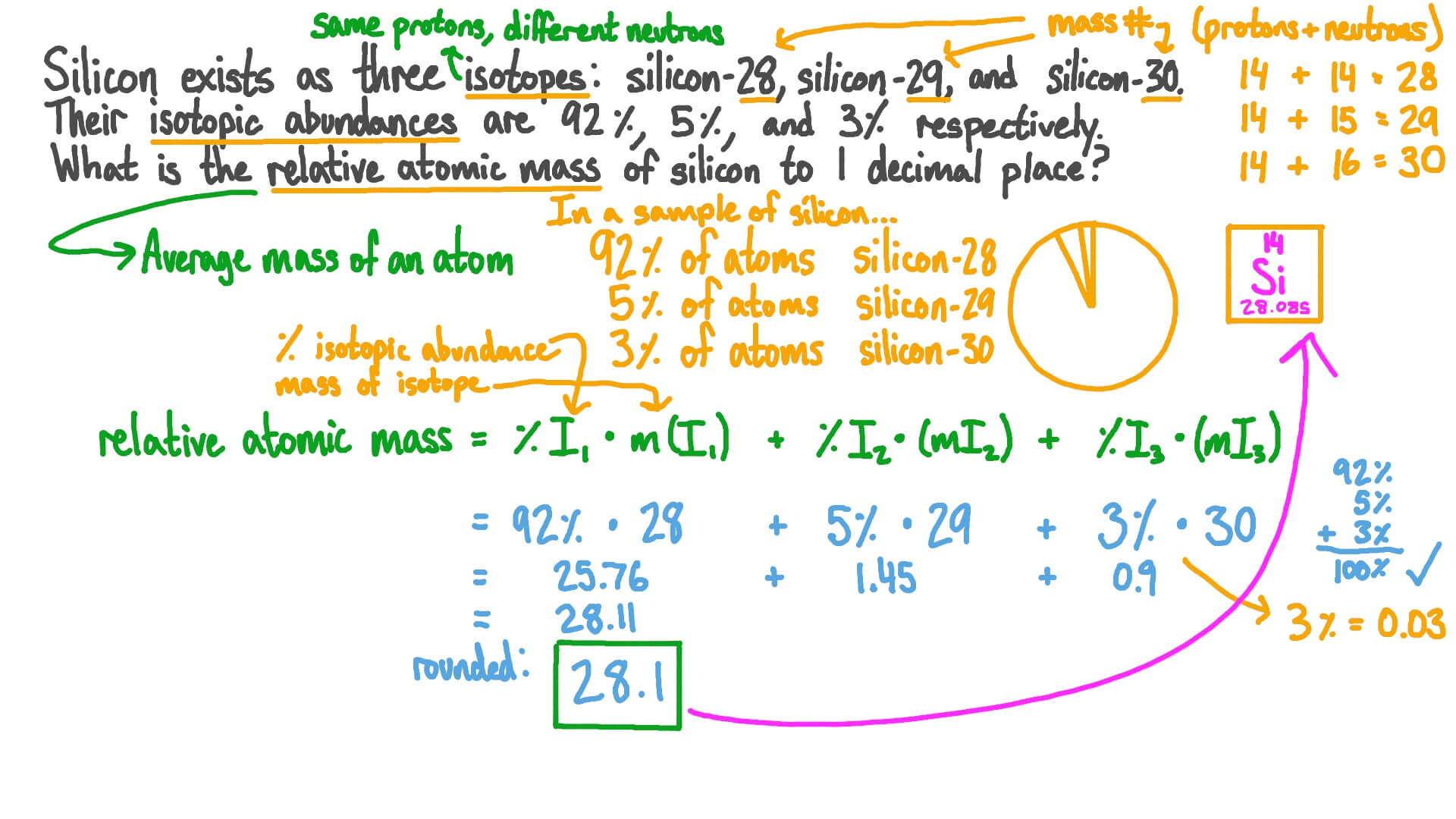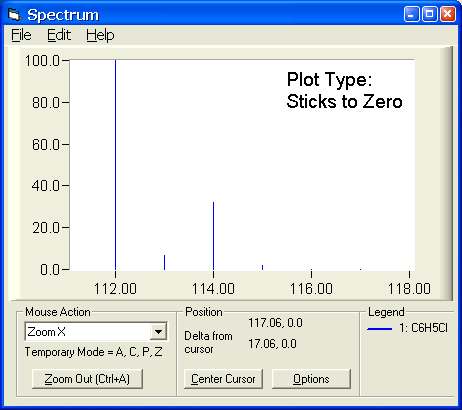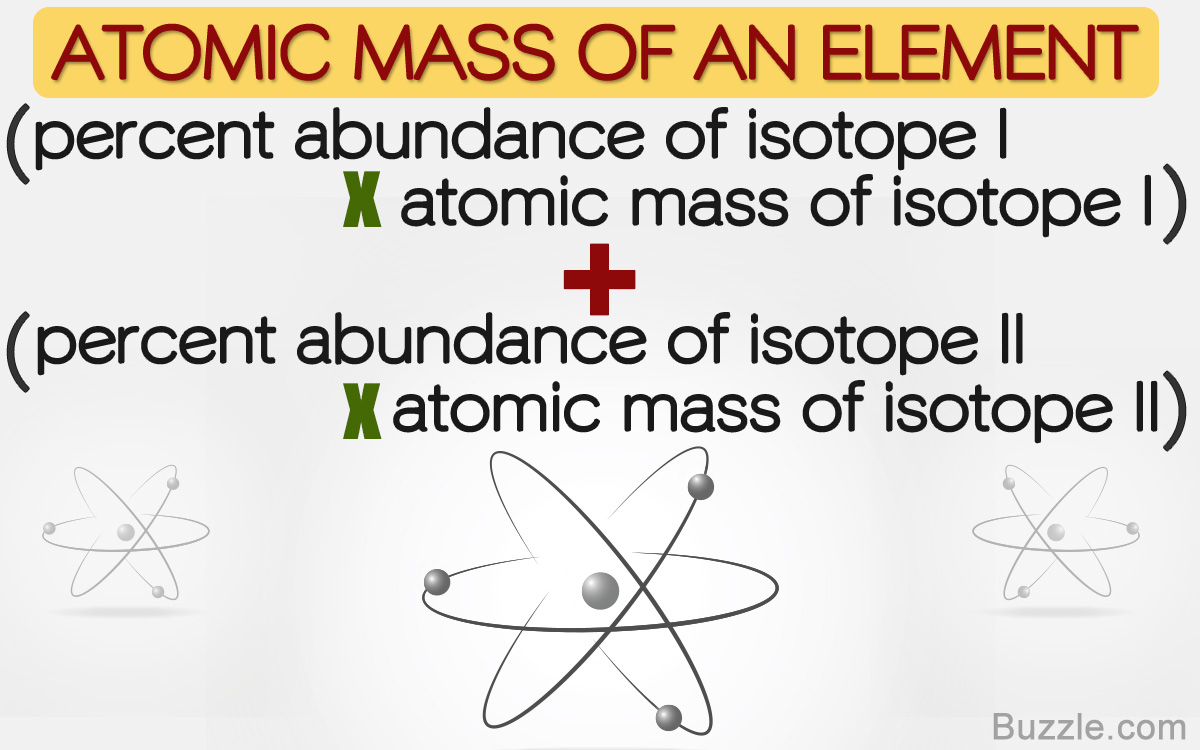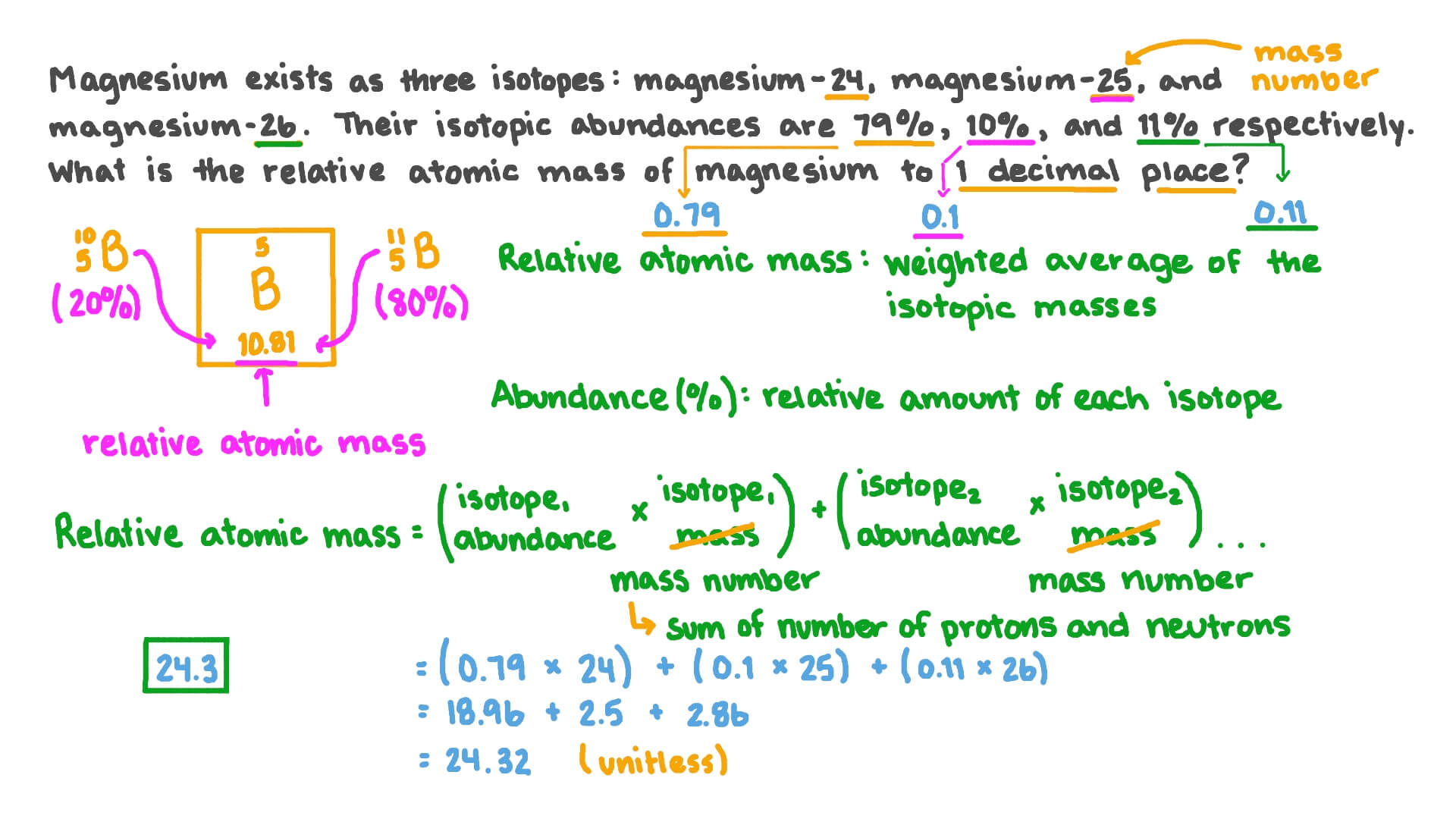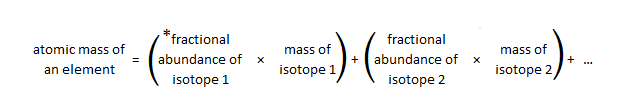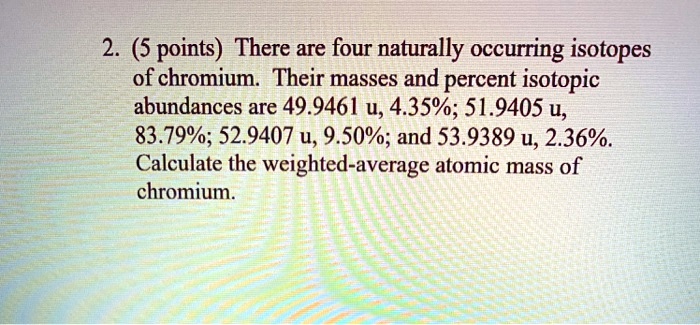
SOLVED: 2. (5 points) There are four naturally occurring isotopes of chromium Their masses and percent isotopic abundances are 49.9461 u, 4.35%; 51.9405 u, 83.79%; 52.9407 u, 9.50%; and 53.9389 u, 2.36% Calculate the weighted-average atomic mass of ...
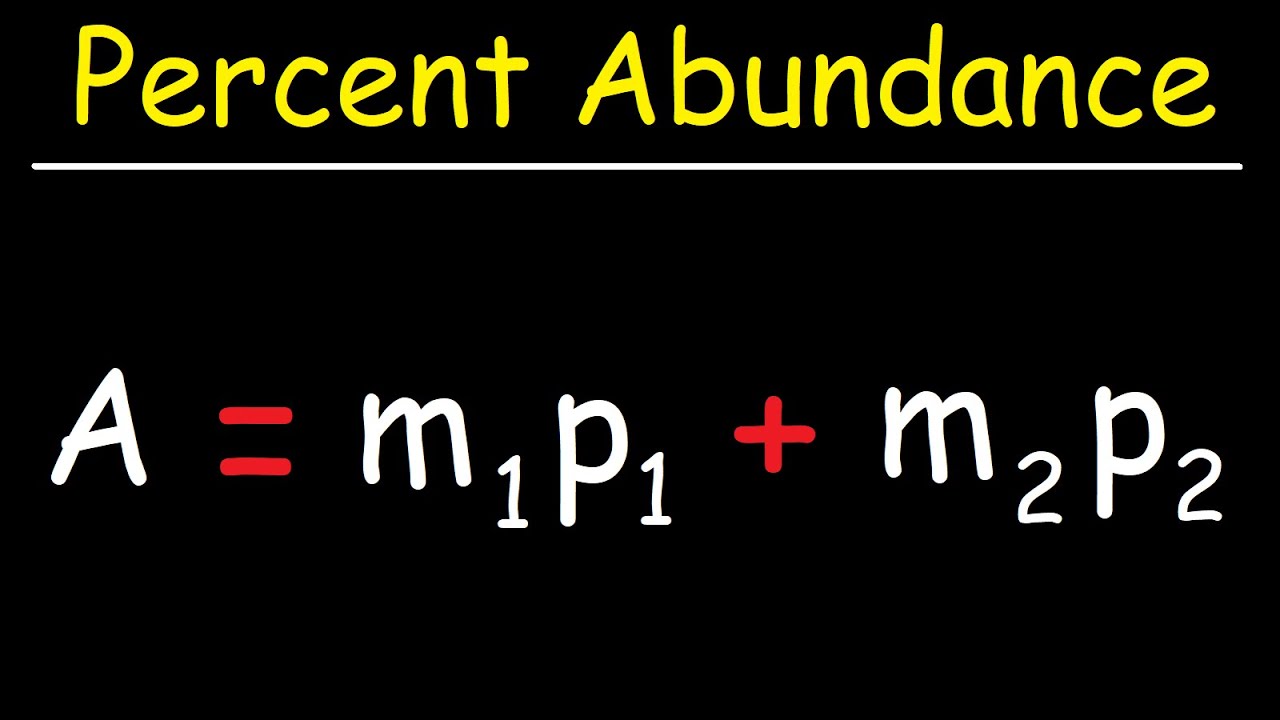
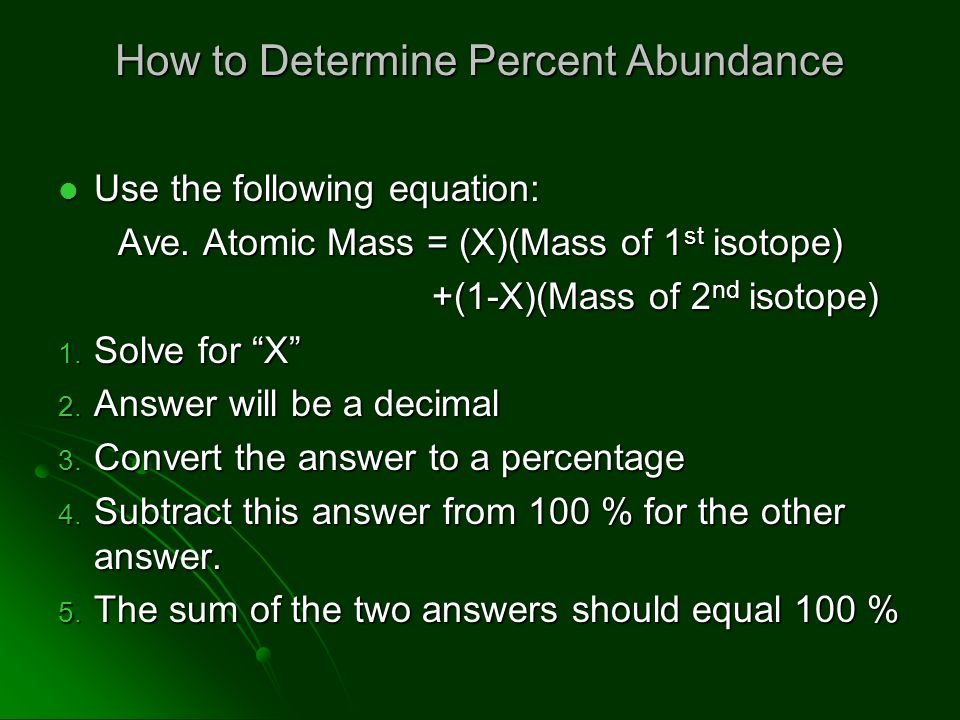
How would you calculate the relative abundance for two isotopes when the relative atomic mass is given? - Quora

How to Solve for Percent Abundance of Isotopes Examples, Practice Problems, Step by Step Explanation - YouTube